HIV-1 Limiting Antigen (LAg) Avidity EIA Kit, 192 Tests
Catalog #: 92001
An in-vitro quantitative limiting antigen (LAg) avidity enzyme immunoassay for distinguishing recent HIV-1 infections from those which are long-term.
This test uses US CDC developed technology and is designed for surveillance purposes such as estimating HIV-1 incidence in a population, monitoring and evaluating HIV intervention programs, and recognizing those high-incidence populations so that prevention research, vaccine trials, and resources are most appropriately utilized.
This product is for research use only and is not intended for use in diagnostic procedures.
Additional information
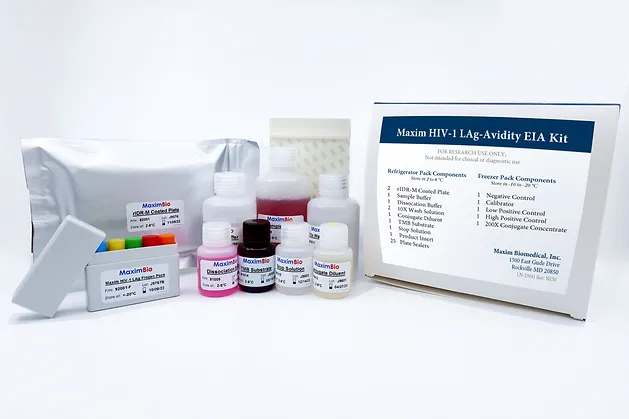
Refrigerator Pack
Recominant gp41 HIV-1 Antigen Coated Microwell Plate (2 Plates - 96 wells x2)
Sample Diluent (1 bottle - 175 mL) Reagents & Buffer Solutions
Dissociation Buffer (1 bottle - 27 mL)
Conjugate Diluent (1 bottle - 27 mL)
10X Wash Solution (2 bottles - 100 mL)
TMB Substrate (1 bottle - 27 mL)
Stop Solution (1 bottle - 27 mL)
Plate Sealers (1 package - 25 plate sealers)
Freezer Pack
Negative Control (1 vial - 50 μL)
Calibrator (1 vial - 50 μL)
Low Positive Control (1 vial - 50 μL)
High Positive Control (1 vial - 50 μL)
200X Conjugate Concentrate (1 vial - 180 μL)
Format: ELISA
Sample Type: Serum/Plasma
Test/Kit: 192 Tests
Storage Temperature: 2-8°C (Refrigerated Pack) & -20°C (Freezer Pack)
FOR USE UNDER FDA EMERGENCY USE AUTHORIZATION (EUA) ONLY
Catalog #: 92001
Description: HIV-1 Limiting Antigen (LAg) Avidity EIA Kit (192 Tests/Kit)
To place an order or to request additional information, please contact our support team.
